Development and Pathologies of Neuromuscular Circuits
Our goal is to understand how muscles are built during development, what dictates their specific shapes, and how they are repaired after injury in adults, in healthy or pathological contexts.
We study the mechanisms driving morphogenesis of skeletal muscles and of the associated motor circuits, and try to understand how dysfunction of these mechanisms, during development or in adults, can cause neuromuscular pathologies. A common theme in these studies is that we focus on cell interactions at the interface between distinct cell types, with our favorite players being motor neurons, muscle cells, and connective tissues. We currently explore interactions between connective tissue cells and muscle cells 1) during embryonic development and 2) during adult skeletal muscle regeneration, in healthy and pathological contexts (see our recent review, Helmbacher & Stricker, 2020).
Publications
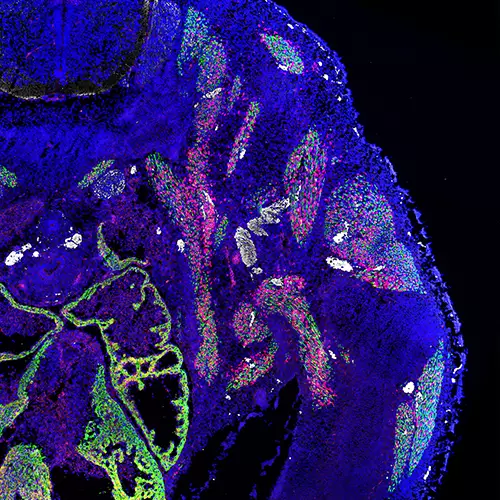
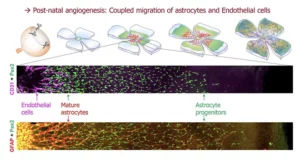
Astrocyte-intrinsic and -extrinsic Fat1 activities regulate astrocyte development and angiogenesis in the retina
Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis
Requirement of FAT and DCHS protocadherins during hypothalamic-pituitary development
Characterization of Etv4GFP and Etv5RFP reporter lines in the context of fibroblast growth factor 10 signalling during mouse embryonic lung development
Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system
Tissue-specific activities of the Fat1 cadherin cooperate to control neuromuscular morphogenesis.
Astrocyte-intrinsic and -extrinsic Fat1 activities regulate astrocyte development and angiogenesis in the retina
Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis
Requirement of FAT and DCHS protocadherins during hypothalamic-pituitary development
Tissue cross talks governing limb muscle development and regeneration
Characterization of Etv4GFP and Etv5RFP reporter lines in the context of fibroblast growth factor 10 signalling during mouse embryonic lung development
Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system
Tissue-specific activities of the Fat1 cadherin cooperate to control neuromuscular morphogenesis.
Correlation between low FAT1 expression and early affected muscle in facioscapulohumeral muscular dystrophy
Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool.
Identification of variants in the 4q35 gene FAT1 in patients with a facioscapulohumeral dystrophy-like phenotype.
Celsr3 is required in motor neurons to steer their axons in the hindlimb.
Plasticity versus specificity in RTK signalling modalities for distinct biological outcomes in motor neurons
gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance.
Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb.
Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline.
The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration.
Targeting of the EphA4 tyrosine kinase receptor affects dorsal/ventral pathfinding of limb motor axons.
News
Join the IBDM for your internship!
Seeking for your Master internship? The IBDM seems like the right place to do it? Check out our offers.
The Helmbacher team identified the Fat1 Cadherin as a novel regulator of retinal vascular integrity, and investigated the underlying mechanisms.
During this master project (M2) the student will apply the above method to obtain proof of principle that targeting selected candidate genes can efficiently induce or suppress intramuscular adipose tissue formation.